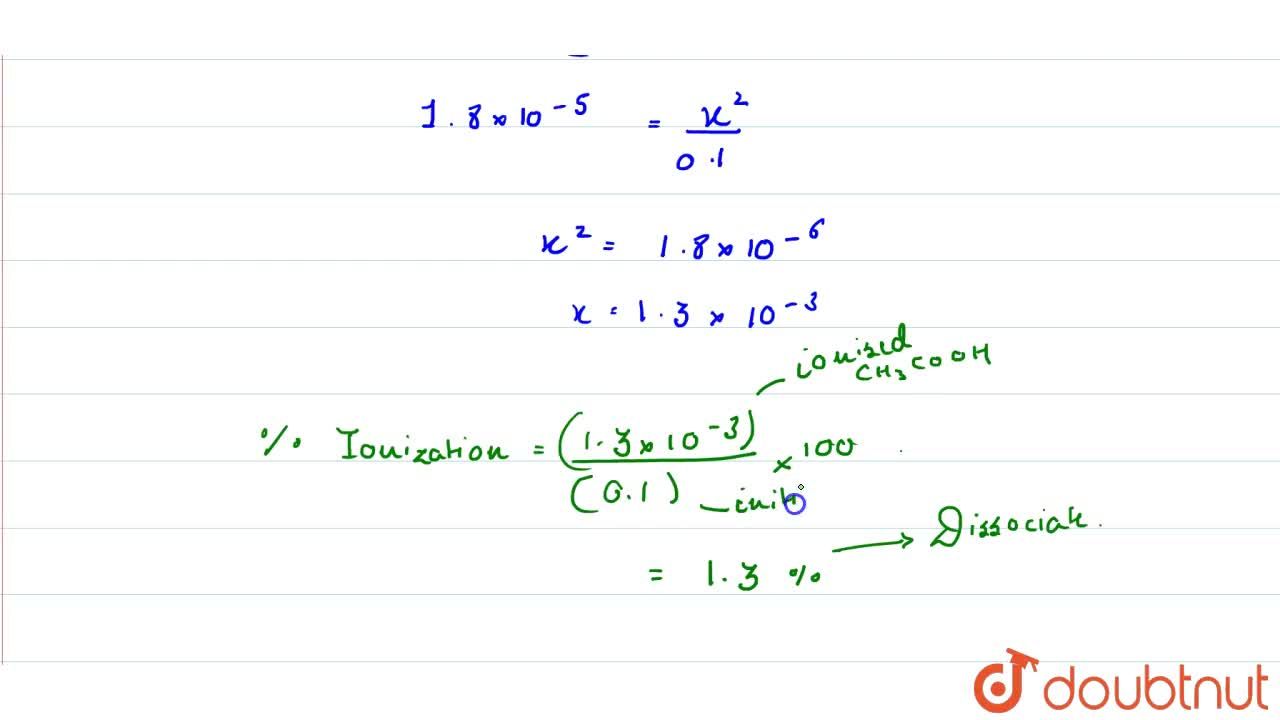
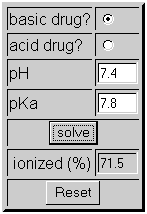
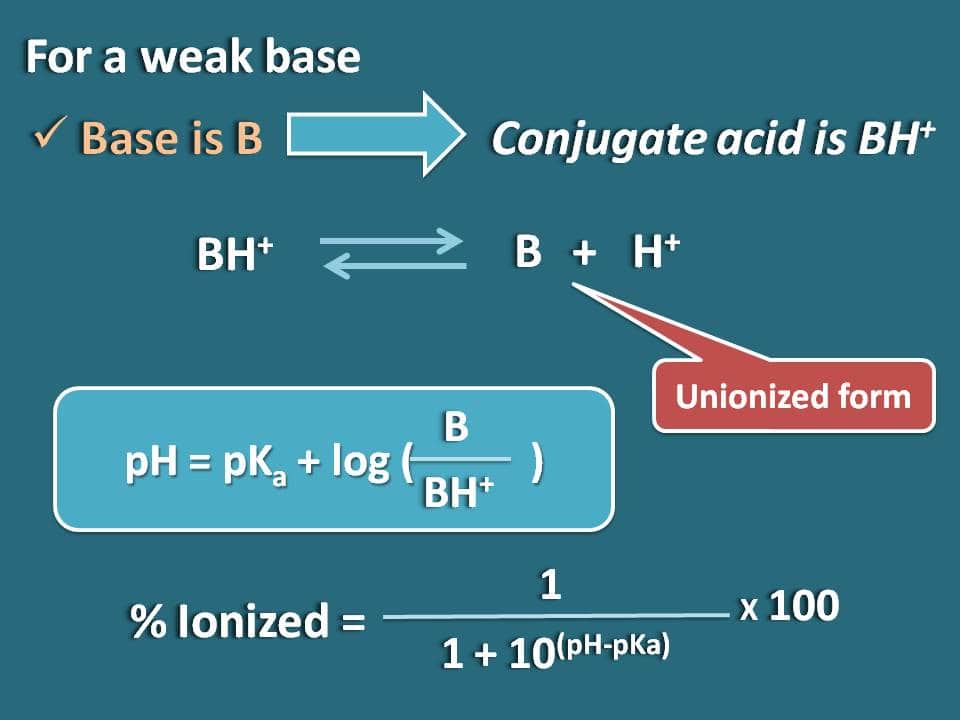
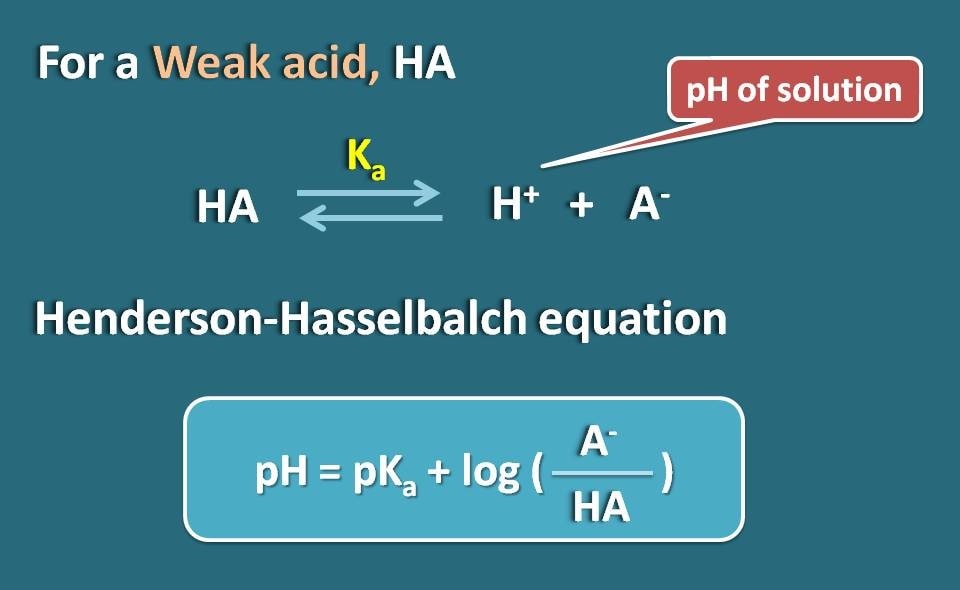
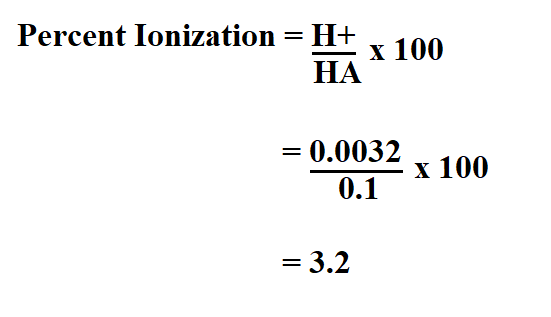
A 0.01 M solution of acetic acid is 1.34 % ionized (degree of dissociation = 0.0134 ) at 298 . What is the ionization constant of acetic acid.

Calculate the degree of ionization of 0.05 M acetic acid if its PKa value is 4.74. How is the degree of dissociation affected when its solution also contains (a) 0.01M (b) 0.1M
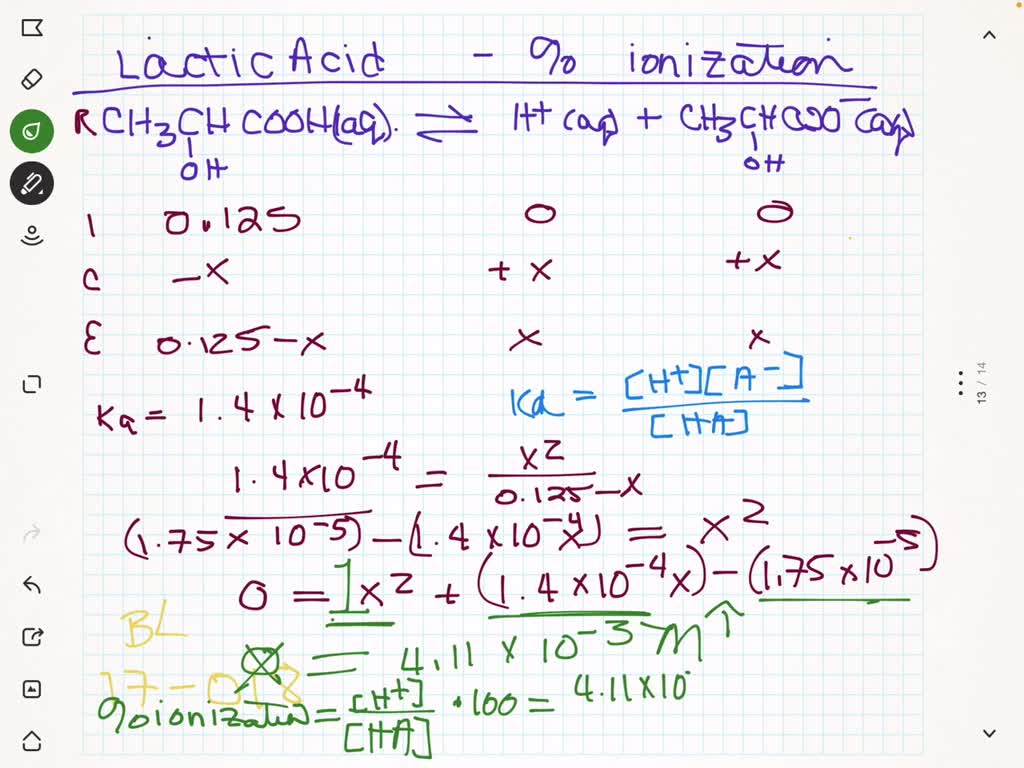
SOLVED:(a) Calculate the percent ionization of 0.125 M lactic acid (Ka=1.4 ×10^-4). (b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora
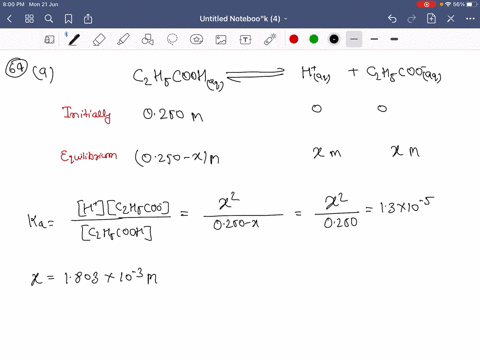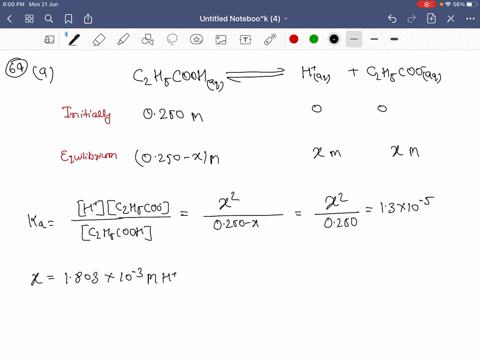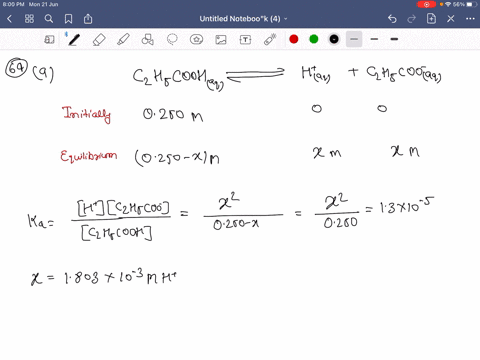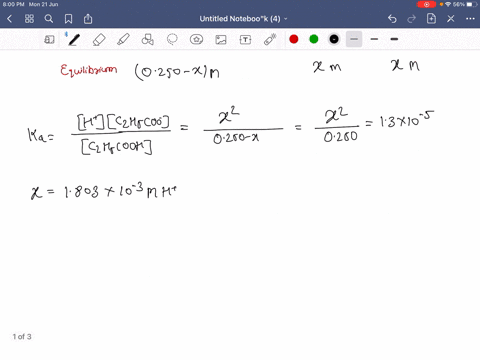
SOLVED:Calculate the percent ionization of propionic acid (C2 H5 COOH) in solutions of each of the following concentrations (Ka is given in AppendixD): (a) 0.250 M,(𝐛) 0.0800 M, (𝐜) 0.0200 M .