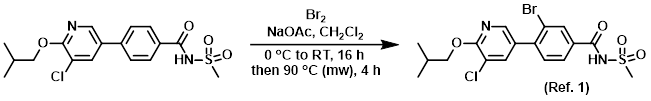Les additifs alimentaires de meilleure qualité No CAS 127-09-3 Naoac/acétate de sodium anhydre/acétate de sodium - Chine Acétate de sodium de la poudre blanche, Food Grade Acétate de sodium

Growth of S. salsiraiae IM408 under different sodium acetate (NaOAc)... | Download Scientific Diagram

Sodium acetate catalyzed tandem Knoevenagel–Michael multicomponent reaction of aldehydes, 2-pyrazolin-5-ones, and cyano-functionalized C–H acids: Facile and efficient way to 3-(5-hydroxypyrazol-4-yl)-3-aryl-propionitriles - ScienceDirect
![Sodium Acetate-promoted Oxa-Michael-Aldol [3+2] Annulation Reactions: Facile Access to the Fused Heterocycle | Bentham Science Sodium Acetate-promoted Oxa-Michael-Aldol [3+2] Annulation Reactions: Facile Access to the Fused Heterocycle | Bentham Science](https://www.eurekaselect.com/images/graphical-abstract/ccat/7/1/006.jpg)
Sodium Acetate-promoted Oxa-Michael-Aldol [3+2] Annulation Reactions: Facile Access to the Fused Heterocycle | Bentham Science

Scheme 1. (a) Br2/NaOAc-buffer, r.t., overnight, 81%. (b) Pd(PPh3)4... | Download Scientific Diagram
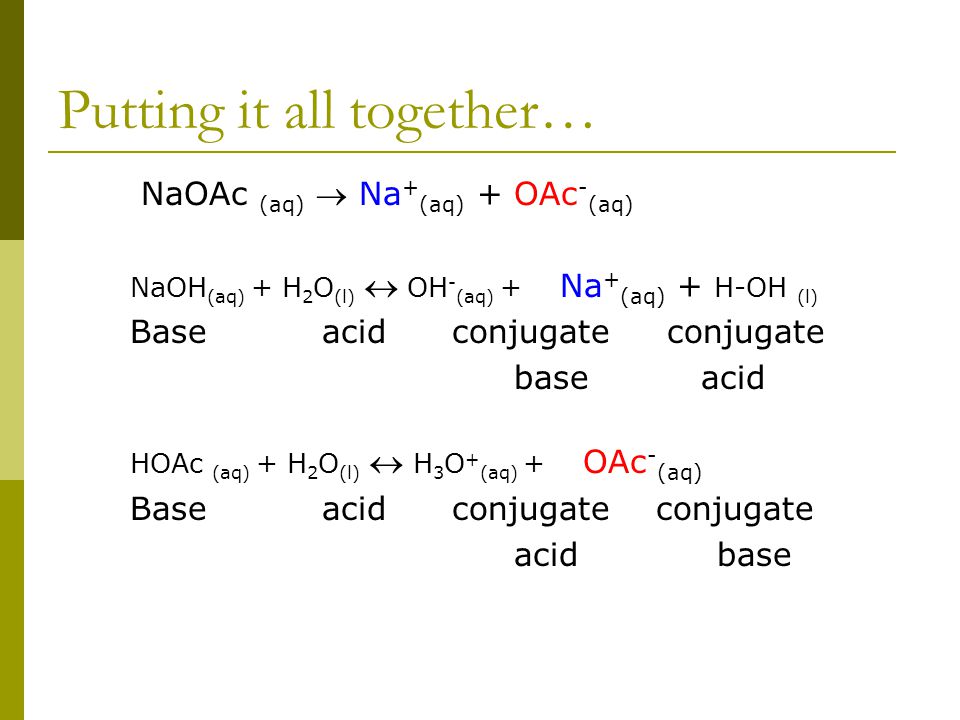
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora

Iodine–sodium acetate (I2–NaOAc) mediated oxidative dimerization of indolizines: an efficient method for the synthesis of biindolizines - ScienceDirect

If sodium acetate is a weak acid and does not readily dissociate in water or completely and a strong electrolyte is defined as the oppposite how come the answer is B and
![Using sodium acetate for the synthesis of [Au(NHC)X] complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/D0DT02240C Using sodium acetate for the synthesis of [Au(NHC)X] complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/D0DT02240C](https://pubs.rsc.org/image/article/2020/DT/d0dt02240c/d0dt02240c-u1_hi-res.gif)
Using sodium acetate for the synthesis of [Au(NHC)X] complexes - Dalton Transactions (RSC Publishing) DOI:10.1039/D0DT02240C

SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow

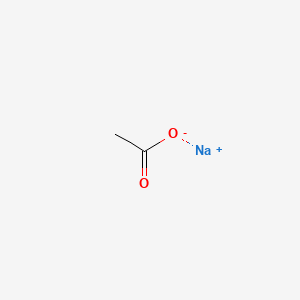
Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.
![Using sodium acetate for the synthesis of [Au(NHC)X] complexes - Dalton Transactions (RSC Publishing) Using sodium acetate for the synthesis of [Au(NHC)X] complexes - Dalton Transactions (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/D0DT02240C)