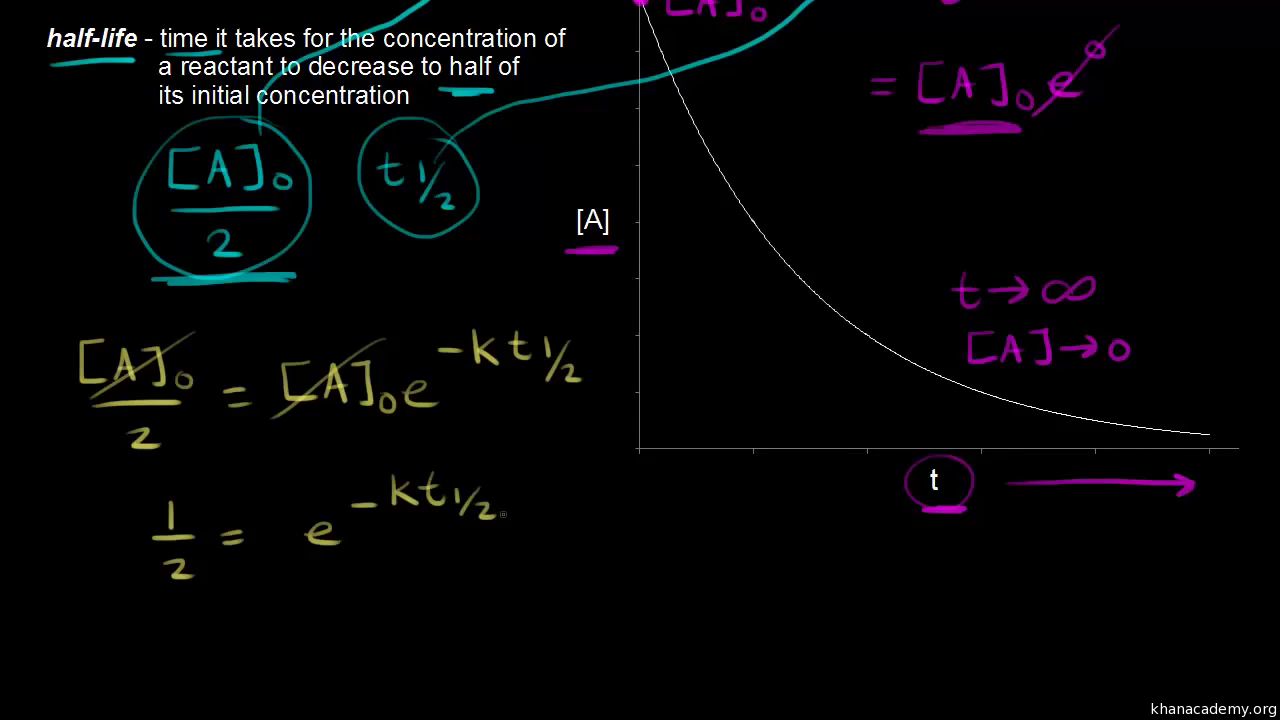
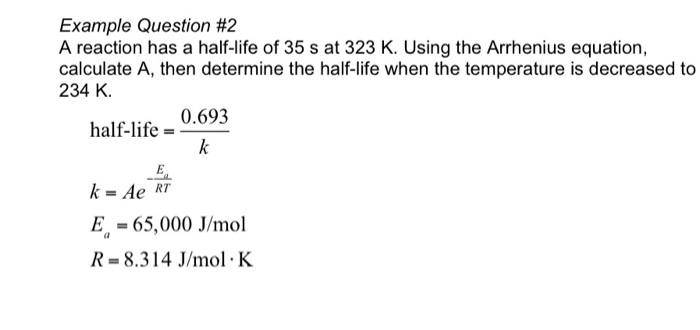
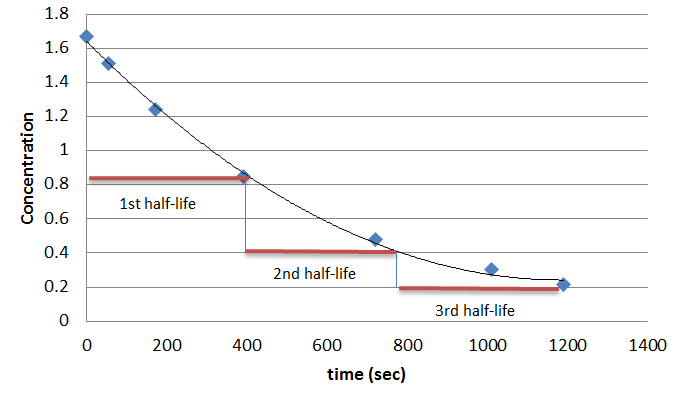
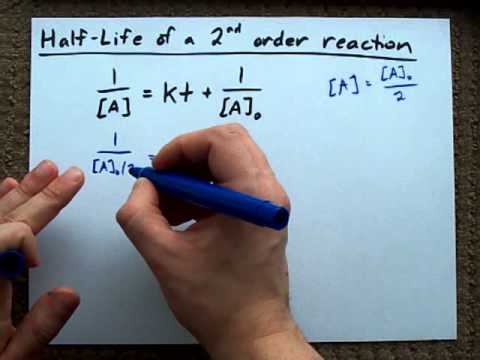
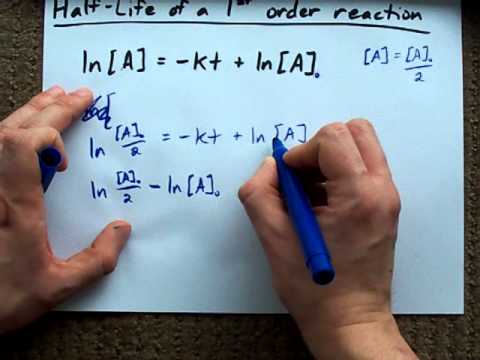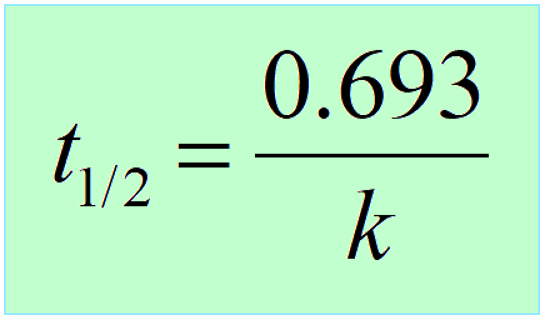The formula of half life for an nth order reaction involving reactant A and n ≠ 1 is - Sarthaks eConnect | Largest Online Education Community

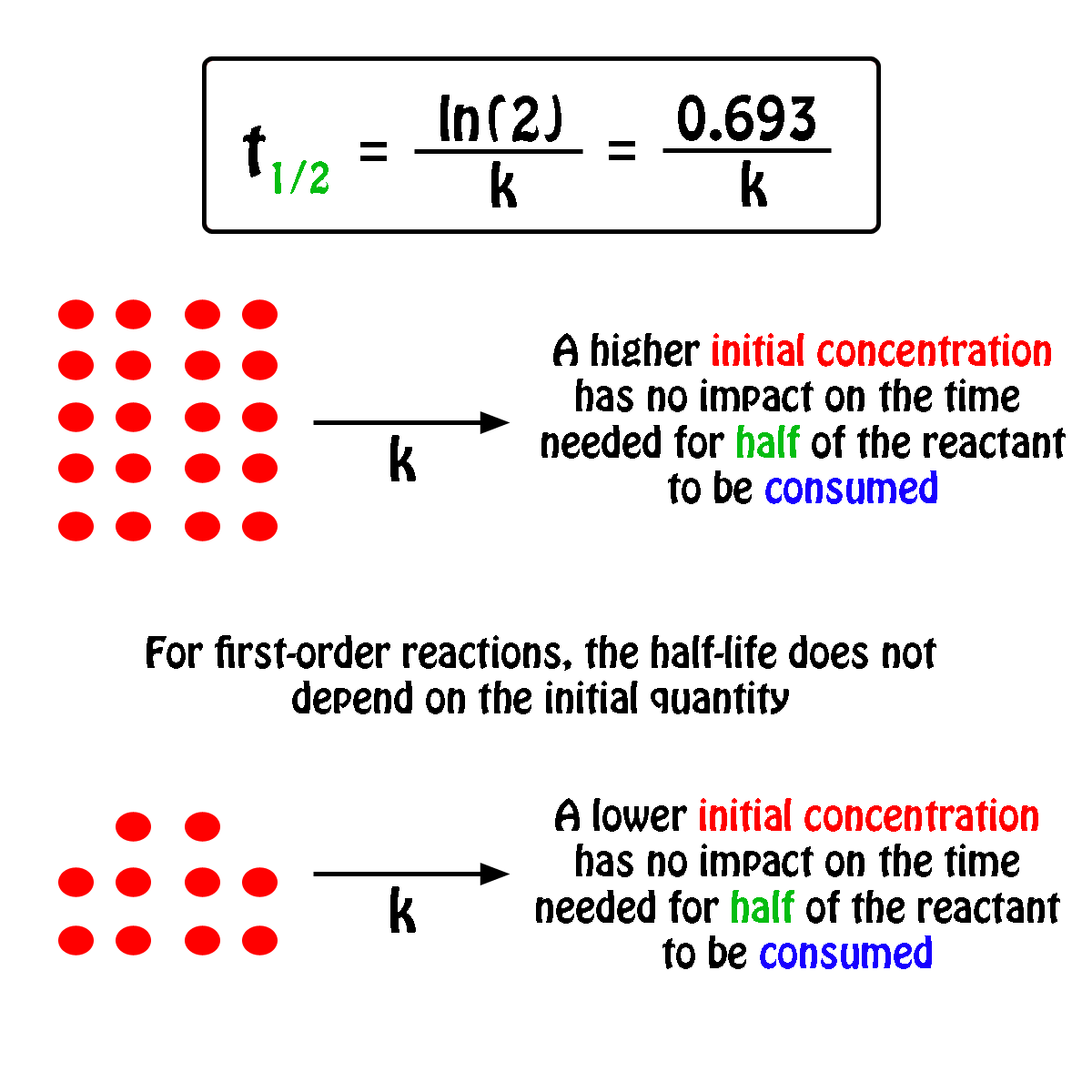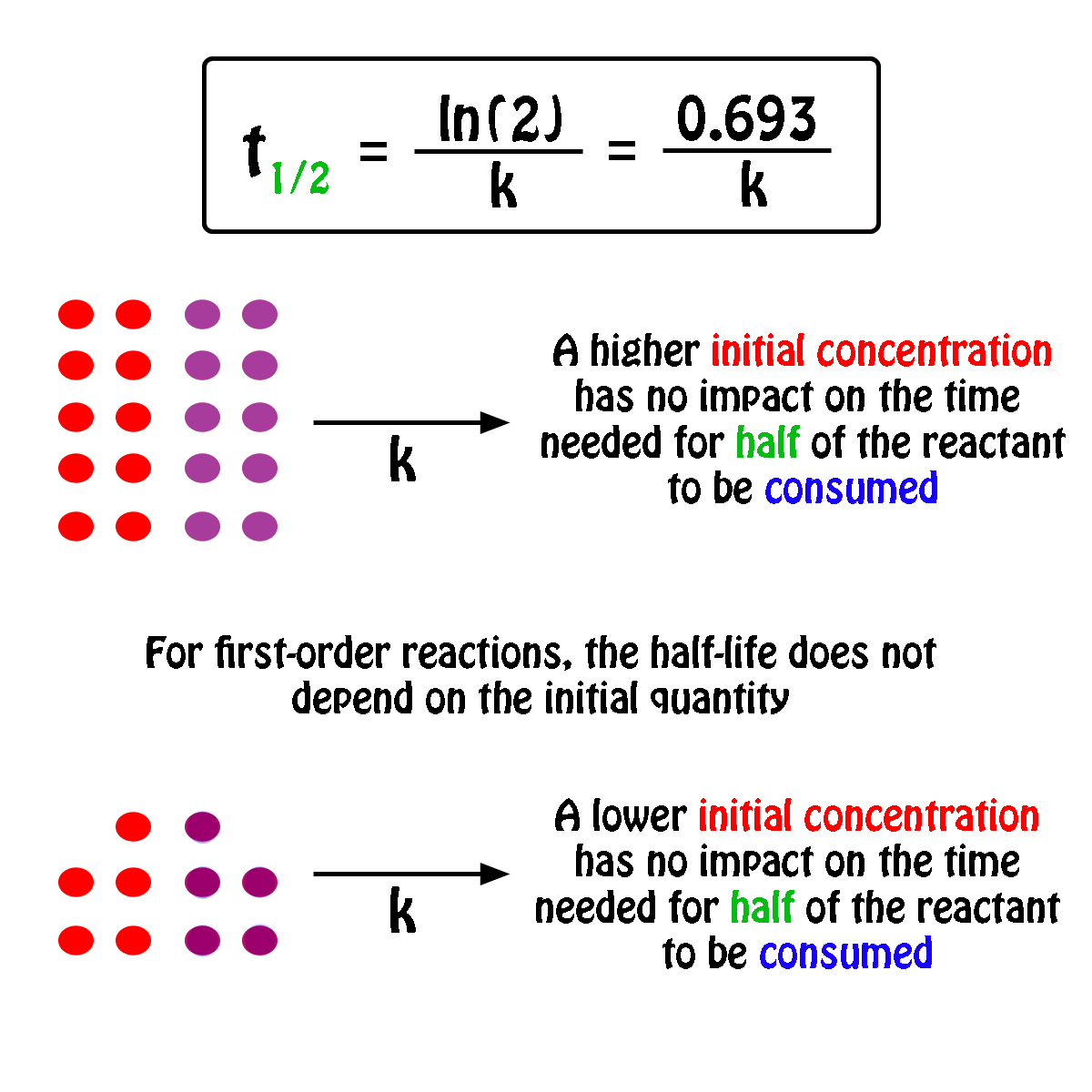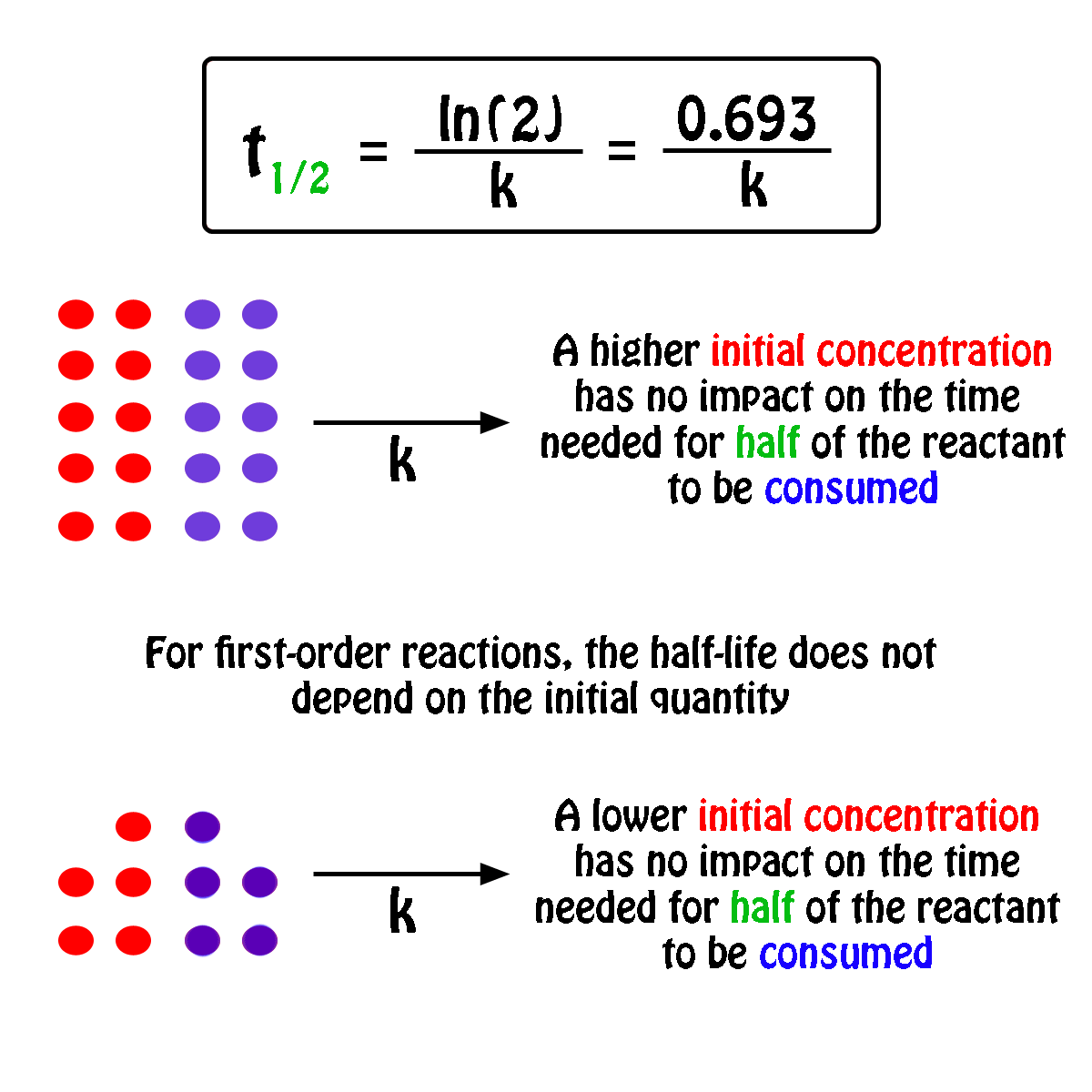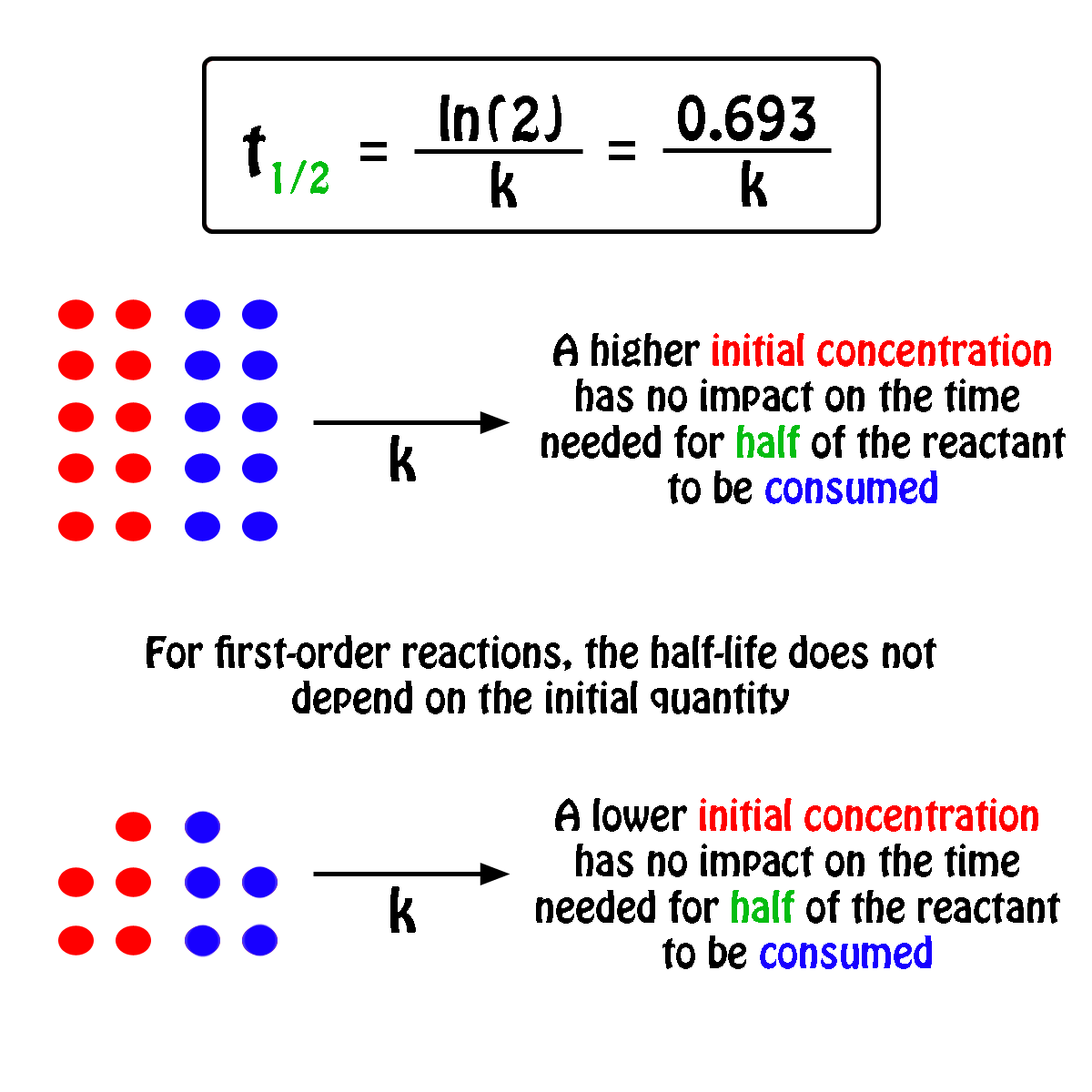
Calculate the half life of a first order reaction from their rate constants given below : a. 200s^(-1),b. 2 mi n^(-1),c.4years^(-1)

Calculate the half life of a first order reaction from their rate constants given below:(a) 200 s^-1 ; (b) 2 min^-1 ; (c) 4 year^-1 .