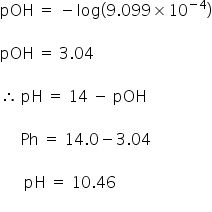
SOLVED: 1. Calculate the pH if you added 3 mL of 0.1 M HCL to a) 97 mL of pure water at pH 7, and b) 100 mL of phosphate buffer (0.063
Calculate pH of [HS^–], [S^2–], [Cl^–] in a solution which is 0.1 M HCl & 0.1 M H2S given that Ka1 (H2S) = 10^–7, Ka2 (H2S) = 10^–14 - Sarthaks eConnect | Largest Online Education Community
![The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ]. The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].](https://dwes9vv9u0550.cloudfront.net/images/4298277/0914b99c-8837-49a9-86f7-3cbcdb1ec4a6.jpg)
The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH would be:[Note : pKa for CH3COOH = 4.74 and log 2 = 0.301) ].

What is the pH of the solution in which 25 ml of 0.08M HCl when added to 25ml of 0.1M NaOH and final solution is diluted to 500 ml. Answer is 11 .

Calculate the pH of the resultant mixture: a. `10 mL` of `0.2M Ca(OH)_(2)+25 mL` of `0.1 M HCl` ... - YouTube

Calculate the pH of solution obtained by mixing 10 ml of 0.1M HCl and 40ml of 0.2 M H2SO4 - Brainly.in
pH of 0.1 M CH3COOH solution is 3 at 25 degree celcius . if limiting molar conductivity of CH3COO and H+ are 40 and 350 S cm2 mol . the molar conductance at 25 degree for 0.1M CH3COOH

What is the pH of a solution in which 25.0 mL of the 0.1 M NaOH is added to 25 mL of 0.08 M HCl and final solution is siluted to 500 mL ?
Calculate the pH of solution obtained by mixing 10 mL of 0.1 M HCl and 40 ml of 0.2 M H2SO4. - Sarthaks eConnect | Largest Online Education Community